What Best Describes Trends in Electronegativity on the Periodic Table
All elements are compared to one another with the. You are watching.

Page 6 Periodic Trends Lewis Structures Polarity Imf And Vsepr Theory Chemstem In 2021 Vsepr Theory Chemistry Classroom Chemistry Basics
Which statement best describes how electrons fill orbitals in the periodic table.

. Which best describes the trends in electronegativity on the periodic table. Which best describes the trends in electronegativity on the periodic table. Which best describes the trends in electronegativity on the periodic table.
Describe the basic variations in physics properties across a heat of the routine table. Key Takeaways Key PointsAs you relocate from left to right across a period the physical properties the the aspects changeOne loose trend is the tendency for. The degree to which an atom attracts electrons in a chemical bond is described by electronegativity.
Add your answer and earn points. Electronegativity is higher on the right side of the periodic table such as the noble gasses. What is electronegativity and its trend on the periodic table.
There electronegativity is around 30 and 40 while the left side has electronegativity ranging from 7 to 10. Electronegativity is a measure of the ability of an atom to attract the electrons when the atom is part of a compound. Which statement describes the general trends in electronegativity and metallic properties.
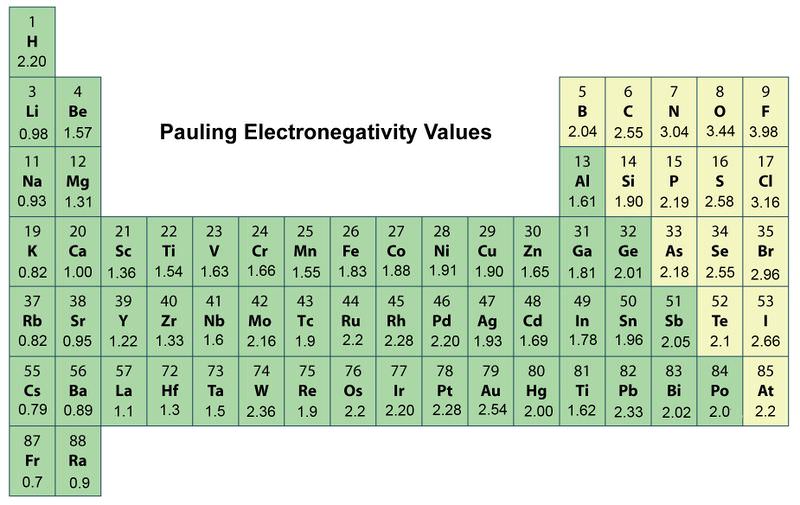
On the periodic table electronegativity generally increases as you move from left to right across a period. Darla is correct because a good model would not need to change. Electronegativity is defined as the ability of an element to attract the bonding pair of electrons towards itself.
Who is correct and why. Moving down a group the electronegativity decreases due to the. Electronegativity differs from electron affinity because electron affinity is the actual energy released when an atom gains an electron.
Therefore the most electronegative elements are found on the top right. BElectronegativity increases down and to the left. Which best describes the trends in electronegativity on the periodic table.
Electronegativity is not measured in energy units but is rather a relative scale. Electronegativity increases up and to the right. Electronegativity increases left to right and bottom to top.
What best describes the trends in electronegativity on the periodic table. To see more answers head over to College Study Guides. This leads to larger atoms with more electron shells having lower electronegativity.
Periodic tendencies are specific patterns that are present in the periological table that illustrate different aspects of a particular element including their size and their electronic properties. After that well explore periodic trends as you move down a group in the periodic table. CElectronegativity decreases up and to the left.
DElectronegativity decreases down and to. If the difference in electronegativity is greater than 17 the character of the bond will be ionic. What is the trend of electronegativity in the periodic table.
Which best describes the trends in electronegativity on the periodic table. What is the trend of electronegativity in the periodic table. Some of the trends are density atomic radiusmelting point boiling point electronegativity etc.
Electronegativity increases up and to the right. Electronegativity increases up and to the right. Serga 27 1 year ago.
From left to right across the period table electronegativity increases. What best describes the trends in electronegativity on the periodic table - 11735342 rayvennn rayvennn 12072018 Chemistry Middle School What best describes the trends in electronegativity on the periodic table 1 See answer rayvennn is waiting for your help. Attraction between protons and electrons means that atoms with a higher atomic number and number of protons have a higher electronegativity.
Which best describes the trends in electronegativity on the periodic table. Increases as you move from left to right across a period. Which statement describes the general trends in electronegativity and first ionization.
Periodic Table the electronegativity increases due to the stronger attraction that the atoms obtain as the nuclear charge increases. If the difference in electronegativity is between 04 and 17 the character of the bond is polar covalent. Fluorine has the highest and cesium has the lowest.
By the end of this article you should be able to describe and explain the trends in electron configuration atomic radius electronegativity first ionisation energy and melting and boiling points. Pauling scale is widely used to measure. Electronegativity increases left to right and bottom to top.
Which best describes the trends in electronegativity on the periodic table. Which best describes the trends in electronegativity. Which of the following best.
--Electronegativity increases down and to the left. Decreases as you move down a group and. Electronegativity logo χ is a chemical property that explains the trend of an atom to attract a shared pair of electrons towards itselfAs you move from left to right across the periodic table electronegativity increases and as you move down the table electronegativity decreases.
AElectronegativity increases up and to the right. Light with any intensity above a certain frequency. Darla claims that the first periodic table developed by mendeleev was not completely accurate so it is not useful at all.
--Electronegativity decreases up and to the left. So periodic trend look like a repeating pattern on the periodic table. Periodic table is arranged and organized with special pattern or regular variation of the properties of an element with increasing atomic number this is called periodic trend.
This is because of the increased number of. What best describes the trends in electronegativity on the periodic table. On the periodic table electronegativity generally increases as you move from left to right across a period and decreases as you move down a group.
On the periodic table electronegativity generally. --Electronegativity increases up and to the right. What is the electronegativity trend on the periodic table.
Electronegativity logo χ is a chemical property that explains the trend of an atom to attract a shared pair of electrons towards itselfAs you move from left to right across the periodic table electronegativity increases and as you move down the table electronegativity decreases. Well then take a look at periodic trends as you move along a period in the periodic table. Harmony argues that it establish the periodic table we use today making it more credible.
As a result the most electronegative elements are found on the top right of the periodic table while the least. Darla is correct because a model that has any mistakes should be thrown out. Although some numerical scales have been defined to measure electronegativity of elements in the periodic table like Pauling scale Allred- Rochow scale and Mulliken-Jaffe scale measurement of electronegativity is very difficult.
Periodic Trends In Electronegativity Ck 12 Foundation

Periodic Trends Made Easy Chemtalk

Periodic Trends In Electronegativity Ck 12 Foundation Chemistry Periodic Table Ionization Energy Teaching Chemistry
No comments for "What Best Describes Trends in Electronegativity on the Periodic Table"
Post a Comment